南湖新闻网讯(通讯员 马召玉)近日,我校韩鹤友教授团队与新加坡南洋理工大学赵彦利教授、华中科技大学同济医学院附属协和医院副主任医师梁华庚合作,针对临床上常用的光声试剂在注入肿瘤病人体内后一直保持常开(always on)状态的早期诊断及治疗瓶颈,设计了一种原位纳米酶放大的近红外II肿瘤精准治疗平台,实现了对乳腺癌的化学动力学和光热联合治疗。相关研究成果以“In Situ Nanozyme-Amplified NIR-II Phototheranostics for Tumor-Specific Imaging and Therapy”为题在Advanced Functional Materials发表。
近红外二区(NIR-II)肿瘤光疗技术,由于其较强的穿透深度、较高的空间分辨率和无创伤性等优点,在纳米医学中具有巨大的应用前景。但是,目前“常开”光疗剂往往有许多不良副作用,阻碍了其临床试验的进展。为了克服这一瓶颈,研究团队开发了一种原位纳米酶放大显色纳米反应器,将3,3',5,5'-四甲基联苯胺(TMB)和超小型PtAu纳米颗粒负载于金属有机框架,用于特定的乳腺癌肿瘤治疗,同时保护了正常组织不受损伤。作为一种智能光声诊断试剂,构建的纳米反应器通过H2O2激活和酸增强状态,打开光声信号,对肿瘤部位进行术前的精准定位和诊断。作为一种纳米酶,肿瘤组织的特殊微环境引发活性氧对其进行催化损伤,实现化学动力学治疗(CDT)。同时,被氧化的TMB对肿瘤组织进行光热疗法(PTT),实现了CDT和PTT最佳组合的“一体式”联合治疗。本研究中的纳米酶可对肿瘤进行特异性消融,有望在肿瘤治疗中进一步开发智能纳米酶。
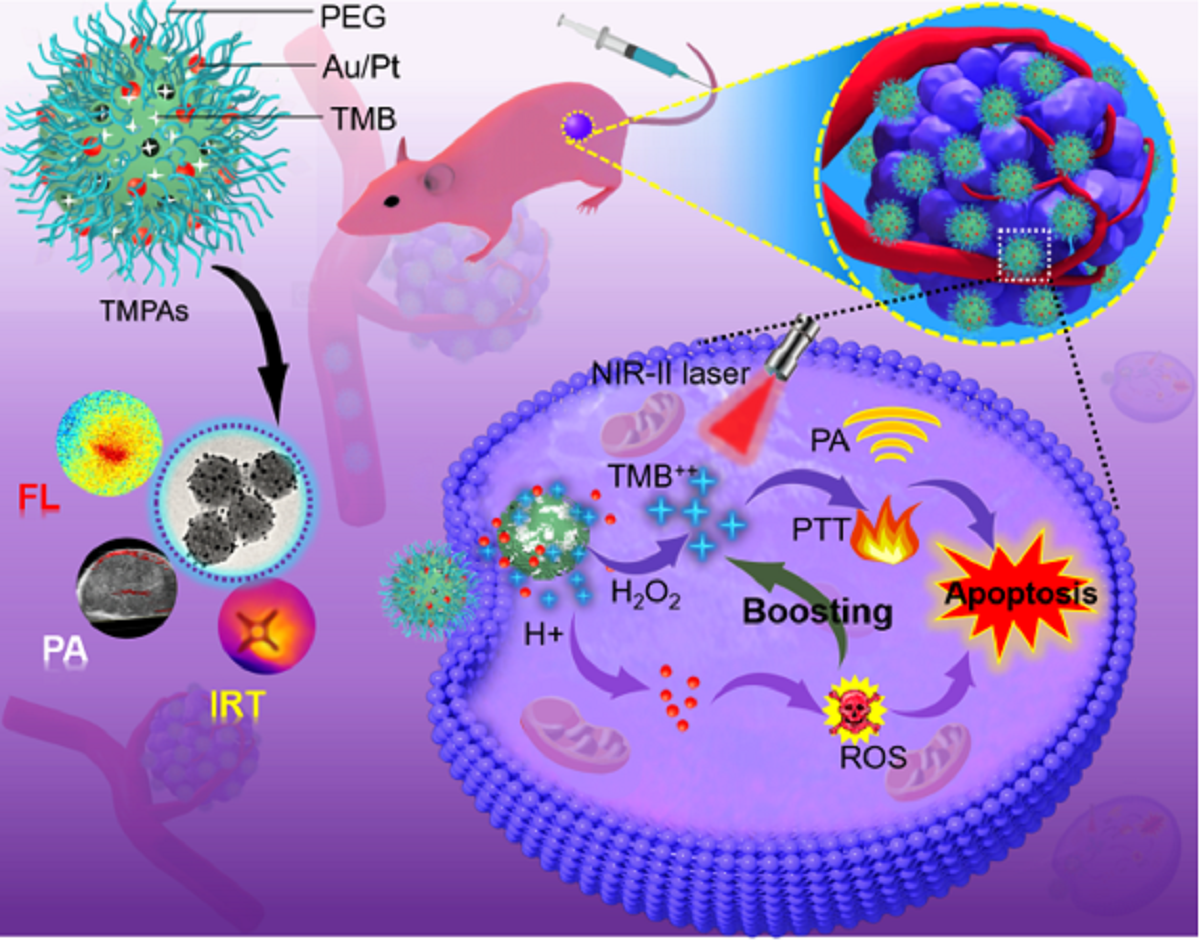
TMPAs的表征

TMPAs的酶活性及催化机理
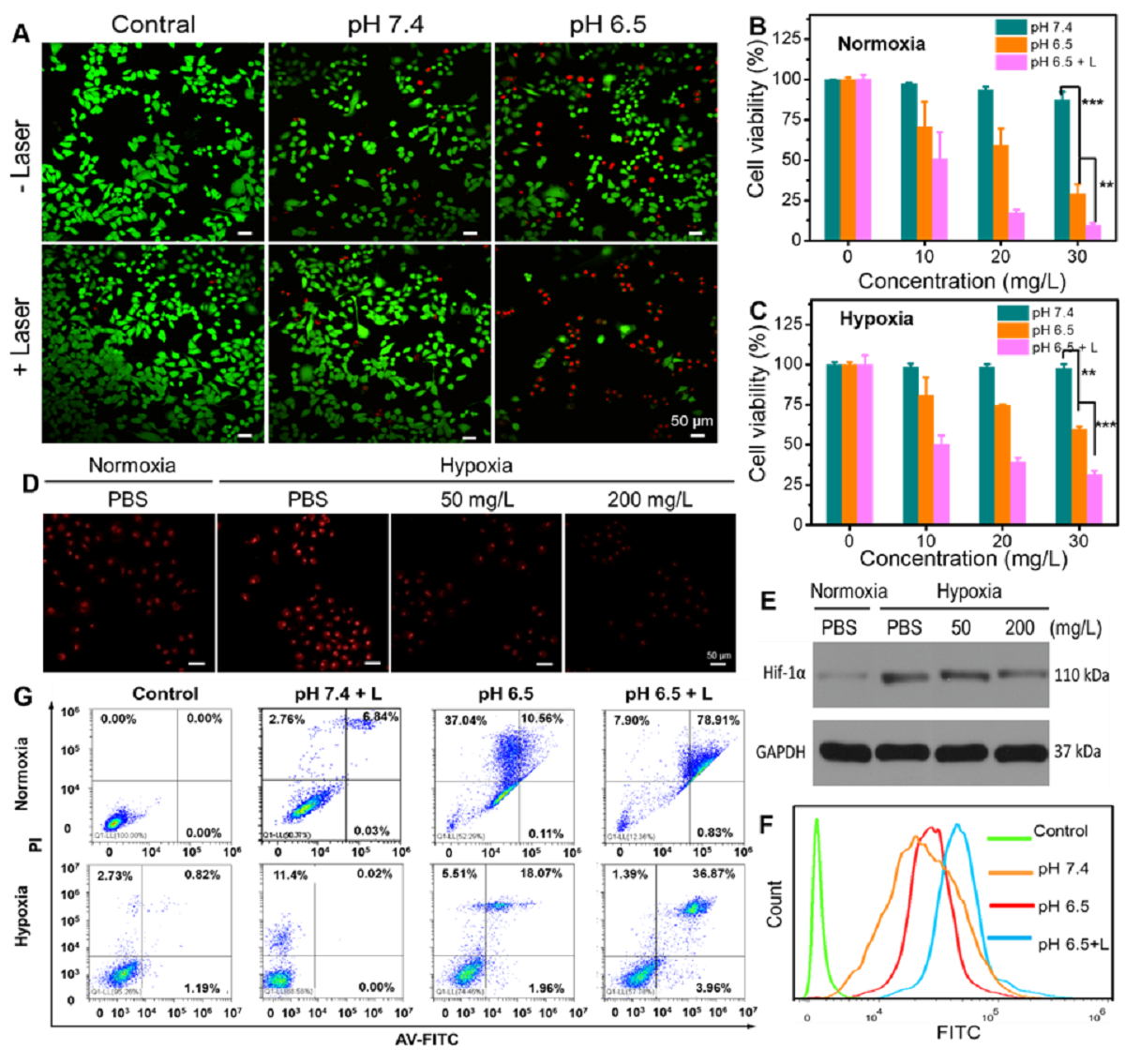
TMPAs的体外协同抗癌作用
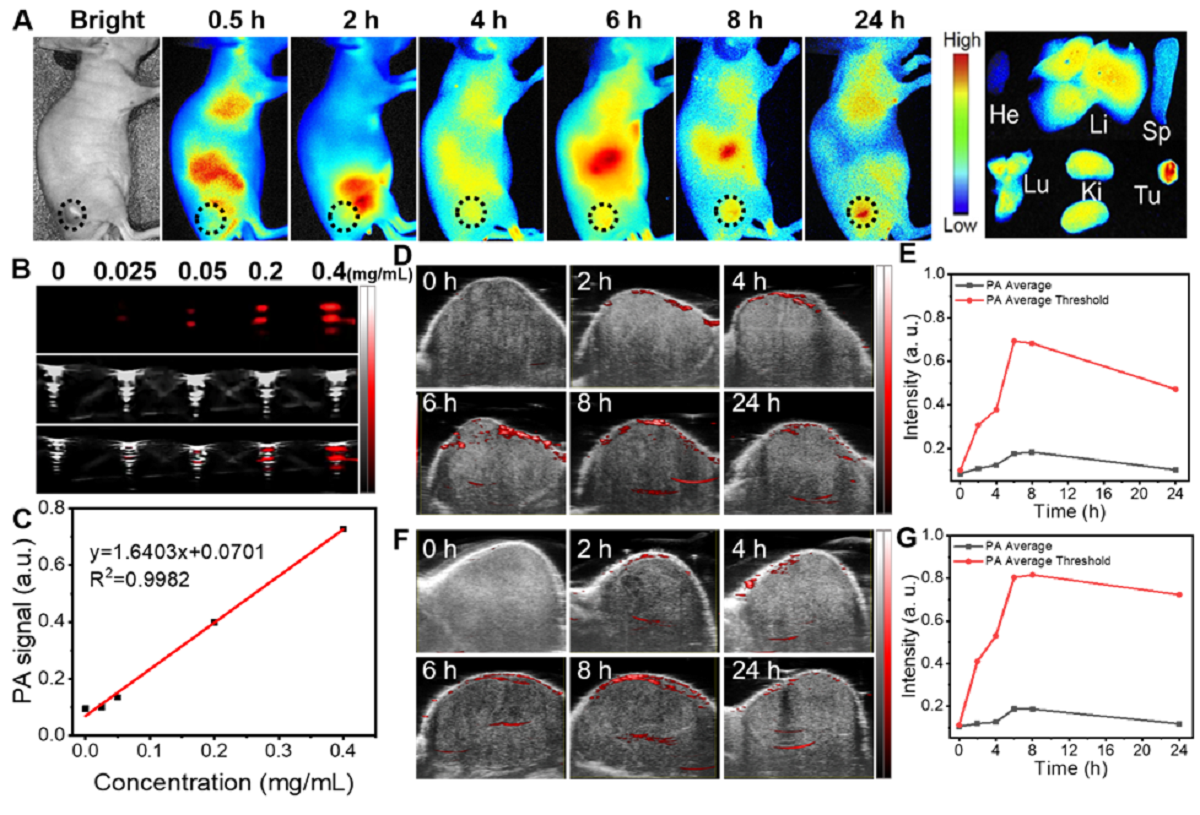
TMPAs的体内光声成像诊断
该研究通过原位纳米酶放大策略,为肿瘤的精准治提供了新的方法。体外和体内试验均表明,该原位纳米酶放大的近红外二区肿瘤精准治疗平台能够准确有效地消融肿瘤,实现了对乳腺癌的CDT-PTT联合治疗,同时对正常组织的非特异性损伤非常小或免受损伤。
我校博士研究生马召玉为该文章第一作者,我校韩鹤友教授、新加坡南洋理工大学赵彦利教授和华中科技大学同济医学院附属协和医院梁华庚医师为该论文的共同通讯作者,我校为成果第一单位。该研究得到了国家自然科学基金等项目的资助。
审核人:韩鹤友
【英文摘要】
The discovery of near-infrared-II (NIR-II) tumor phototheranostics holds a great promise for use in nanomedicine on account of its enhanced penetration depth, high spatial resolution, and noninvasiveness. However, contemporary "always on" phototherapeutic agents often have many undesirable side effects that hinder their clinical trial progress. To overcome this dilemma, an in situ nanozyme-amplified chromogenic nanoreactor by loading 3,3′,5,5′-tetramethylbenzidine (TMB) and ultrasmall PtAu nanoparticles into a metal-organic framework is developed for specific tumor theranostics, leaving normal tissues unharmed. As an intelligent photoacoustic diagnostic agent, the as-constructed nanoreactor remains silent until they enter the tumor site (H2O2-activated and acid-enhanced conditions) and turns on photoacoustic signal to render a preoperative tumor diagnosis. As a nanozyme, the special microenvironment of the tumor tissue is used to initiate its catalytic damage by reactive oxygen species for chemodynamic therapy (CDT). More importantly, the TMB is oxidized, and the subsequent photothermal therapy (PTT) can be realized leading to an optimal combination of CDT and PTT to concurrently fight obstinate cancers. The present “all-in-one” phototheranostics utilize nanozyme-augmented NIR-II agents for specific tumor ablation, which are promising for further development of intelligent nanozymes in the tumor therapy.
论文链接:https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.202103765
